This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemists create industrially important alkyl amines from dinitrogen and alkenes

A critical chemical bond can be assembled using dinitrogen (N2)—a molecule freely available in the air around us—chemists at RIKEN have shown in a new article published in Nature.
This demonstration has the potential to make the synthesis of industrially important compounds such as polymers and drugs much more energy efficient.
Dinitrogen is everywhere, making up nearly 80% of the air we breathe. But despite dinitrogen's high availability, it is challenging to use it directly in chemical reactions. That's because the molecule's strong triple bond needs to be severed.
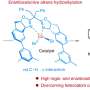
For applications such as the synthesis of alkyl amines for making drugs and polymers, dinitrogen is first split in two to generate ammonia, in an additional step called the Haber–Bosch process. The easily accessible alkene component also has to be pre-activated by converting it into an alcohol or carboxylic acid. These extra steps add time and inefficiency to the process.
They have another disadvantage. "Synthesizing these nitrogen and carbon sources also consumes a lot of energy," notes Takanori Shima of the RIKEN Center for Sustainable Resource Science.
Researchers are searching for better alternatives to this approach. "It would be better to directly use dinitrogen and alkenes to synthesize alkyl amines under mild conditions," he adds. "But such a reaction was unknown and expected to be highly challenging."

Previously, Shima and his collaborators had discovered a way to overcome these challenges using titanium polyhydrides, which are chemical complexes of titanium atoms bridged by hydrogen atoms.
"We have found that titanium polyhydride exhibits extremely high reactivity toward stable small molecules such as dinitrogen and benzene," says Shima.
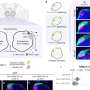
Now, Shima and his team have shown that the multiple titanium–hydride units within titanium polyhydride can work cooperatively to generate alkyl amines from dinitrogen and alkenes.
"When we reacted alkenes with titanium polyhydride, the alkenes were activated—but many titanium–hydride units remained after the reaction," Shima says.
When the team then added dinitrogen, the free titanium–hydride units cooperatively cleaved the dinitrogen molecule, and then clipped the activated carbon and nitrogen species together via a new nitrogen–carbon bond, producing the alkyl amine.
A computational analysis revealed that the key to the reaction is that after activating both substrates, the formation of nitrogen–carbon bonds selectively occurs in the titanium polyhydride framework. That is because the formation of nitrogen–carbon bonds is much more energetically favorable than other possible pathways, such as nitrogen–hydrogen or carbon–hydrogen bond formations.
Shima and his team are now exploring ways to turn this transformation into a catalytic process.
More information: Takanori Shima et al, Hydroamination of alkenes with dinitrogen and titanium polyhydrides, Nature (2024). DOI: 10.1038/s41586-024-07694-5
Journal information: Nature
Provided by RIKEN